- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart
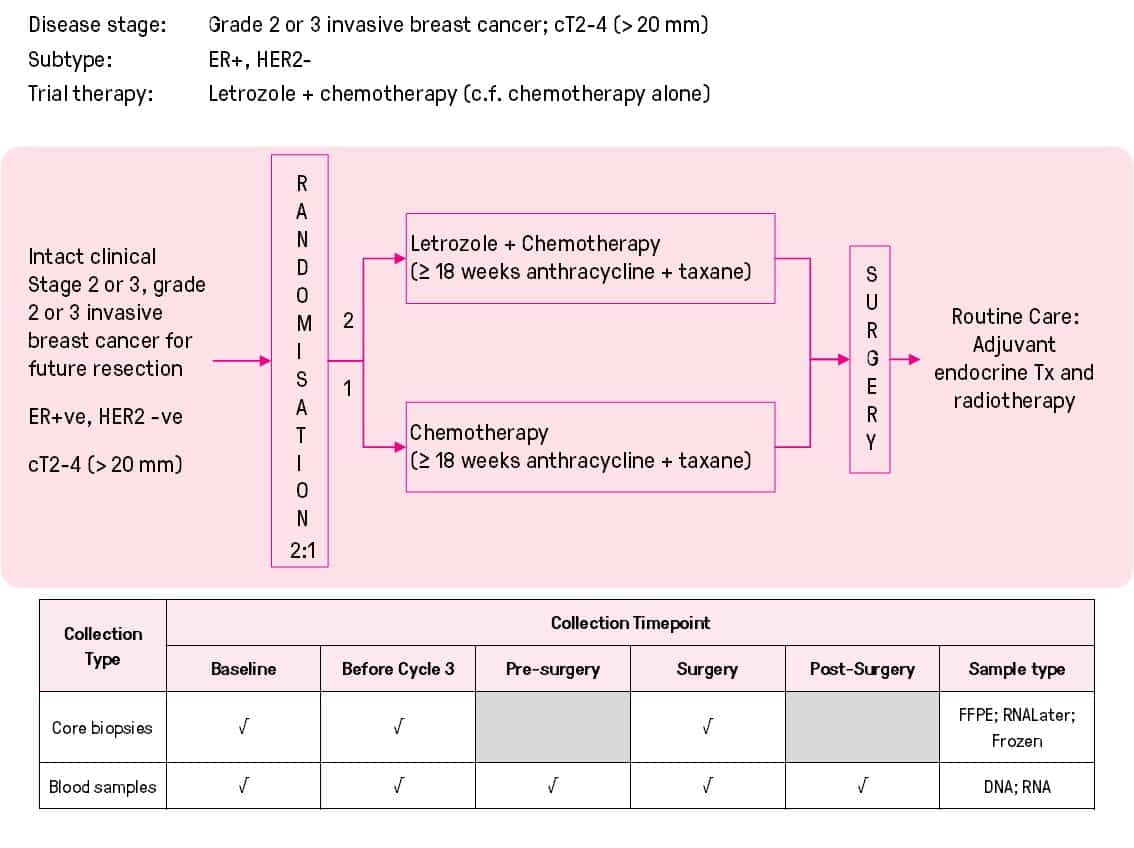
Randomised phase II trial of neoadjuvant chemotherapy +/- concurrent aromatase inhibitor endocrine therapy to down-stage large oestrogen receptor positive breast cancer (ACTRN12614000515695)