- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart
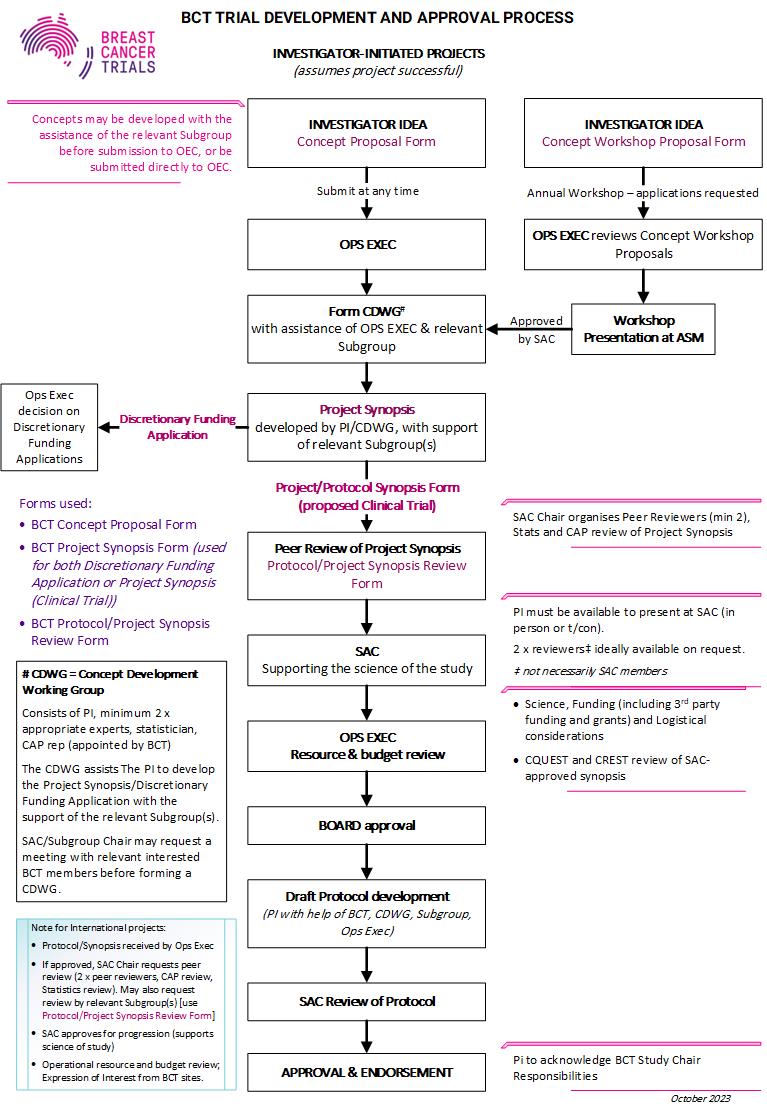
BCT welcomes the submission of concepts from all disciplines relevant to breast cancer care, including medical, surgical or radiation oncology, reconstructive surgery, imaging, pathology, and the disciplines involved in supportive care.
The BCT Subgroups in Supportive Care, Systemic Therapy, Translational Research and Very Early Breast Cancer facilitate the creation of scientifically valid research proposals.
Concepts can be submitted at any time. There is also an annual call for concepts for presentation at the Concept Development Workshop at the BCT Annual Scientific Meeting.
Applications are encouraged from non-BCT members, however, for all projects that are approved for ongoing development, investigators must become BCT members. Click here for further information about BCT membership.
Additional information about the BCT trial development process is available to members via the Research section of the website: Research Development and Funding.
Research Types
Discretionary Funding: A one-off grant of up to $50,000 per year, over 1-2 years.
Clinical Trial Development Funding: Supports longer-term research projects (minimum three years) of high strategic value that are unfunded. Projects must be coordinated by the BCT Trials Department.
Discretionary Funding
Discretionary Funding grants are primarily to support small scale research studies such as Pilot studies that have the potential to lead to BCT-coordinated trials.
Funding may also be considered for sub-studies of protocols BCT is currently involved in, small-scale translational research studies, or projects related to research methodology.
How to Apply
Submit the Concept Proposal Form to the BCT Operational Executive.
The Concept Proposal Form may be submitted to the relevant SAC Subgroup (Supportive Care, Systemic Therapy, Very Early Breast Cancer) for initial review before submission to the Operational Executive.
The BCT Operational Executive reviews the Concept Proposal to ensure that it meets the aims of the BCT research program and identifies the appropriate SAC Subgroup to support development into a Discretionary Funding Application (BCT will provide the Application template on approval of the concept).
An approved Concept Proposal will be developed with a Concept Development Working Group (CDWG), assisted by the relevant SAC Subgroup.
Researchers may already have specialist researchers to form a CDWG, however the Operational Executive will provide guidance if required. The CDWG must consist of at least two specialist area experts, a statistician and a Consumer Advisory Panel representative.
The Investigator and CDWG develops the Concept Proposal into a Discretionary Funding Application.
The Investigator submits the application to the BCT Operational Executive.
For approved applications, the Investigator completes the Discretionary Funding Agreement with BCT and submits regular reports (as required by the Agreement) during the project.
Clinical Trial Development
To be considered for clinical trial development with BCT, projects must:
- Fulfil the definition of clinical or translational research that relates to prior, current or the development of future BCT-led breast cancer clinical trials
- Have the potential to make a significant impact on the care of those affected by breast cancer
- Be feasible as research with significant involvement from BCT, including being coordinated by the BCT Trials Department
- Potentially be eligible for competitive funding e.g. NHMRC, MRFF or Cancer Councils.
How to Apply
Concept Proposal and Synopsis Development
Submit the Concept Proposal Form to the BCT Operational Executive.
The Concept Proposal Form may be submitted to the relevant SAC Subgroup (Supportive Care, Systemic Therapy, Very Early Breast Cancer) for initial review before submission to the Operational Executive.
The BCT Operational Executive initially reviews the Concept Proposal to ensure that it meets the aims of the BCT research program and does not compete with a project that may be in the trials pipeline.
An approved Concept Proposal will be developed into a Project Synopsis (template form provided by BCT on approval of the concept) with a Concept Development Working Group (CDWG), assisted by the relevant SAC Subgroup.
Researchers may already have specialist researchers to form a CDWG, however the Operational Executive/Subgroup Chair will provide guidance if required. The CDWG must consist of at least two specialist area experts, a statistician and a Consumer Advisory Panel representative.
The Investigator and the CDWG develops the Concept Proposal into a Project Synopsis.
The investigator submits the Project Synopsis to the BCT Operational Executive.
Peer Review and SAC Review
The SAC Chair will coordinate peer review of the Project Synopsis; at least two independent experts, a statistician and the CAP will review synopses.
The peer reviews and the Project Synopsis will be presented for discussion and assessment by the Scientific Advisory Committee (SAC). The Investigator will present the project to SAC, including responses to review.
The SAC evaluates the scientific merit and feasibility of project proposals and determines the next steps required to develop the proposal into a Clinical Trial Protocol*.
* Funding options are also considered during this process. If BCT support for a grant application is needed, then BCT support must be confirmed (usually after peer- and SAC-review of the Project Synopsis) and sufficient protocol logistics developed to allow budget formulation. Supply of study treatment or other in-kind support must be confirmed.
Protocol Development
Protocol development is contingent on confirmed funding, supply of study treatment, and approval by the BCT Board of Directors.
Approved projects proceed to Protocol Development, which is coordinated by the BCT Trials Department and supported by the CDWG and relevant SAC Subgroup.
Following protocol endorsement (when the protocol is close to finalisation) by the SAC and Consumer Advisory Panel (CAP), the BCT Trials Department coordinates clinical trial registration and expression of interest with BCT sites; develops patient-related and trial-related documents, data collection and drug management systems; and manages HREC submissions.
BCT Investigator-Initiated Trials
The following clinical trials have been developed via the BCT Clinical Trials Development process:
- PROSPECTIVE
- OLIO
- Breast MRI Evaluation
- CAPTURE
- Neo-N
- EXPERT
- DIAmOND
- CHARIOT
- ELIMINATE
- PROSPECT
- SOLACE
- DOMINO
- SORBET
- LATER
- NeoGem
Discretionary Funding Projects
The following projects have been funded by BCT Discretionary Funding:
- PANTOCIN: A phase II, randomised, double-blinded, placebo controlled, crossover trial to assess Pantoprazoles effectiveness as prophylaxis against delayed chemotherapy-induced nausea and vomiting (CINV) in patients receiving adjuvant breast cancer chemotherapy
- Treatment of Vaginal Atrophy Using Fractional Microablative CO2 Laser in Post-Menopausal Women with Breast Cancer on Aromatase Inhibitors: A Pilot Study
- TROG 14.04: Multicentre Study of Feasibility and Impact on Anxiety of DIBH in Breast Cancer Patients
- Understanding the barriers to, and facilitators of, ovarian toxicity assessment in breast cancer clinical trials which has helped lead to Measuring ovarian toxicity in clinical trials: an American Society of Clinical Oncology research statement
- Genitourinary symptoms in women with breast cancer: what do oncology health professionals think and do about them?
- The Implementation of a Decision Aid for women with early-stage breast cancer considering prophylactic mastectomy: a pilot study
- MDM2 inhibition in combination with endocrine therapy and CDK4/6 inhibition for the treatment of ER-positive breast cancer and BCT Research Blog
- The timeline and quality of life implications of madarosis in patients undergoing cytotoxic chemotherapy for breast malignancy
- Development of a patient decision aid for women with early stage unilateral breast cancer considering contralateral prophylactic mastectomy
- The SEGMENT study: A next generation SEquencing panel for GenoMic alterations for advanced brEast caNcer patienTs and BCT Research Blog
- iPrevent a web-based decision support tool for breast cancer risk assessment and management
- ANZ1302 Aromatase Inhibitor induced musculoskeletal syndrome (AIMSS) and BCT Research Blog