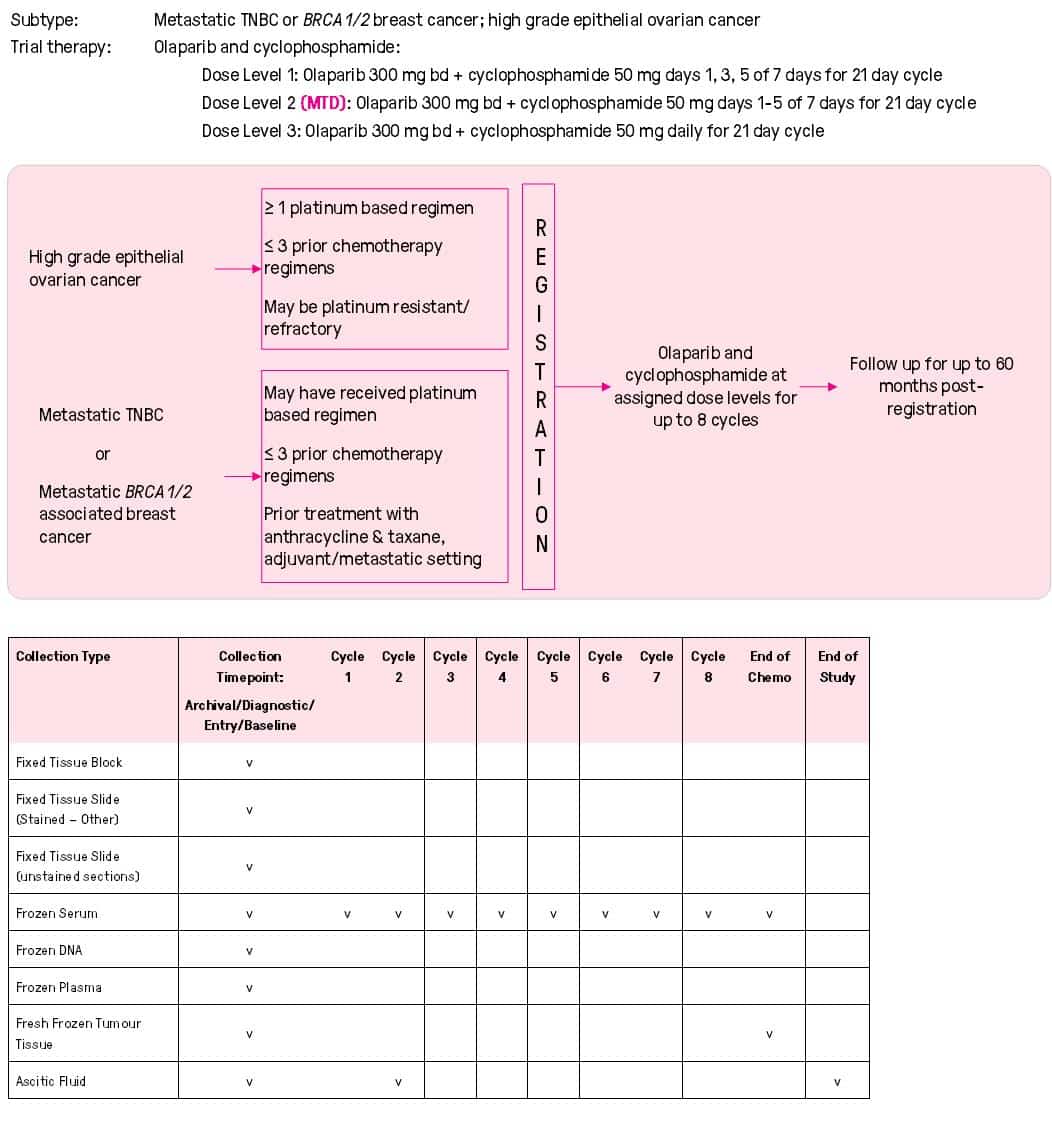
- Research
- 2022-2026 Research Strategy
- Open Clinical Trials
- Closed Clinical Trials
- What is a Clinical Trial?
- Why Participate in a Clinical Trial
- Remote Telehealth Pre-Screening Process
- Research Achievements
- Publications
- Research Development and Funding
- Participating Institutions
- International Collaboration
- BCT Trials & Projects Summary
- Translational Research
- Clinical Fellowship Program
- International Fellowship Support
- Annual Scientific Meeting
- Travel Grants and Awards
- About
- Our Impact
- Fundraise
- Donate
- Researcher Login
- Cart

0
INTERNATIONAL
Total number of trial
participants internationally
32
AUSTRALIA & NEW ZEALAND
Total number of trial participants
in Australia and New Zealand

3
INSTITUTIONS
Total number of participating institutions
in Australia and New Zealand
SOLACE TRANSLATIONAL RESEARCH SAMPLES
SOLACE PUBLICATIONS
2019
Phase 1 trial of olaparib and oral cyclophosphamide in BRCA breast cancer, recurrent BRCA ovarian cancer, non-BRCA triple-negative breast cancer, and non-BRCA ovarian cancer.
Lee CK, Scott C, Lindeman GJ, Hamilton A, Lieschke E, Gibbs E, Asher R, Badger H, Paterson R, Macnab L, Kwan EM, Francis PA, Boyle F, Friedlander M. British Journal of Cancer. 2019; 120(3):279-285, (epub 17 January 2019), Journal
2018
A Phase 1 Trial of Olaparib and Oral Metronomic Cyclophosphamide in Patients with Metastatic BRCA- Associated Breast / Ovarian Cancers or Non-BRCA Associated Triple-Negative Breast Cancer / High Grade Serous Ovarian Cancers. [MOGA 2018 – Poster].
Friedlander M, Scott CL, Lindeman GJ, Gibbs E, Badger HD, Paterson RJ, Macnab L, Zdenkowski N, Kwan EM, Boyle FM, Lee CK, on behalf of Breast Cancer Trials. Asia-Pacific Journal of Clinical Oncology. 2018; 14(53):59, Poster
2017
Phase I study of olaparib (O), in combination with oral cyclophosphamide (C), in patients (pts) with metastatic triple negative breast cancer (TNBC) and recurrent high grade serous ovarian cancer (OVCA).
Lee CK, Scott CL, Lindeman GJ, Gibbs E, Badger HD, Paterson RJ, Macnab L, Kwan EM, Boyle FM, Friedlander M, on behalf of the Australia and New Zealand Breast Cancer Trials Group. Journal of Clinical Oncology. 2017; 35(suppl; abst 1104).[ASCO Annual Meeting], Poster